首先,看了一下谷歌翻译:
Waiver: an act or instance of waiving a right or claim.(放弃权利或要求的行为或实例。)
Exemption: the process of freeing or state of being free from an obligation or liability imposed on others.(摆脱强加于他人的义务或责任的过程或状态。)
从字面意思看,官方给你Waiver,是彻彻底底的放弃要求你做一些事情,这些事情是你本就可以不做的,申请人更主动,豁免更彻底;而给你Exemption,是你经过努力,摆脱了官方规定的义务,而这些事情本应该你完成的,申请人更被动,豁免不豁免在于官方的态度。
其次,这两个词汇是法律词汇,找到一个参考:
https://www.acus.gov/recommendation/regulatory-waivers-and-exemptions
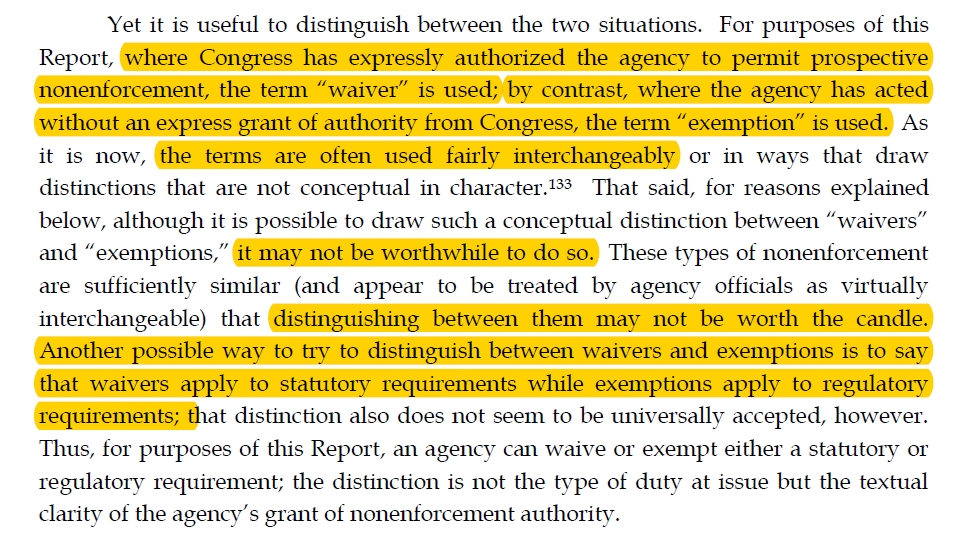
The terms “waiver” and “exemption” carry various meanings in agency practice. For the purposes of this recommendation, when Congress has expressly authorized an agency to excuse a regulated party from a legal requirement, the term “waiver” is used. If an agency is implicitly authorized by Congress to excuse a regulated party from a legal requirement, “exemption” is used. These definitions stem from the report underlying this recommendation. See Aaron L. Nielson, Waivers, Exemptions, and Prosecutorial Discretion: An Examination of Agency Nonenforcement Practices (Nov. 1, 2017) (report to the Admin. Conf. of the U.S.), https://acus.gov/report/regulatory-waivers-and-exemptions-final-report.
当国会明确授权某个机构免除受监管方的法律要求时,将使用“Waiver”一词。 如果国会默示授权某个机构免除受监管方的法律要求,则使用“Exemption”。
可以看到微妙的差别,Waiver更明确更彻底,Exemption有点不情不愿的意味。
上面又给了一个参考文献,里面的解释是:
As explained below, “waiver” is the term that this Report uses when referring to explicit permission to agencies from Congress to not enforce the law.
Also as explained below, “exemption” is the term that this this Report uses when referring to general delegations from Congress to agencies, which the agency then uses to create a nonenforcement scheme—in other words, where the nonenforcement authorization is implicit rather than explicit. This proper terminology is not clearly established in the literature or U.S. Code.
“waiver”是本报告在指国会明确允许各机构不执行法律时使用的术语。
另外“exemption”是本报告在提及国会向各机构的一般授权时使用的术语,然后该机构使用该术语来制定不执行计划,换句话说,不执行授权是隐含的而不是明确的 。 文献或美国法典中没有明确规定这个正确的术语。
第三,看一个FDA的实例:
|
When can I request a waiver from DSCSA requirements? |
An authorized trading partner may request a waiver if the requirements would result in an undue economic hardship or for emergency medical reasons, including a public health emergency declaration pursuant to section 319 of the Public Health Service Act.
|
|---|---|
|
When can I request an exception from DSCSA requirements? |
A manufacturer or repackager may request an exception to the requirements relating to product identifiers if a product is packaged in a container too small or otherwise unable to accommodate a label with sufficient space to bear the information required for compliance with section 582 of the FD&C Act.
|
|
When can I request an exemption from DSCSA requirements? |
An authorized trading partner or other stakeholder may request an exemption for other products or transactions from certain section 582 requirements to maintain public health or is otherwise appropriate.
|
我个人理解以上三点是比较契合的,互为印证,供参考。
非常感谢有心的回答@ggxwtt。
https://acus.gov/report/regulatory-waivers-and-exemptions-final-report.
这个报告非常有帮助,把介绍二者区别的部分放在这里:
——大概意思是:
waiver和exemption是对“违法”的前瞻性授权的行为。前瞻性授权行为又分2种情况,一种是(记作-第一种)国会(congress)创建这个系统或规则,允许agency提供这种前瞻性授权;另外一种是(记作-第二种)国会不直接建立这种系统或规则,而是授权agency来创建自己的程序和规则,agency据此创建了自己的系统或规则,并提供前瞻性授权。
从报告中描述看,waiver对应的是第一种前瞻性授权,exemption对应的事第二种前瞻性授权。也是报告中提到另一种区分二者含义的可能方法“waivers apply to statutory requirements while exemptions apply to regulatory requirements”。但同时报告中也提到没有被大家广发接受,并且区分二者的意义也没有那么大。

建议可参考一个美国论坛上他们本地人的回答,虽非医药领域,但是我认为比较简明切题。 FAA Regs 101: The Difference between an Exemption and a Waiver | Pilots of America
简言之就是 Exemption 倾向于是由于法律规则的客观限制(或者漏洞)使其可免于法律的制约而豁免( an exemption is a type of rulemaking that is granted to a person or entity in order to relieve them from a regulation when no other means to deviate from the regulation applies. ),而 waiver 则是法规专门提出的并且授予的豁免。
这{{threadTextType}}正{{isAdminText}}
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:
