【思考与建议】
根据对中美欧法规总结,EU ASMF可行性最大,CEP和US
DMF可行但操作空间有限,国内个人理解目前是不可行的。
具体依据如下:
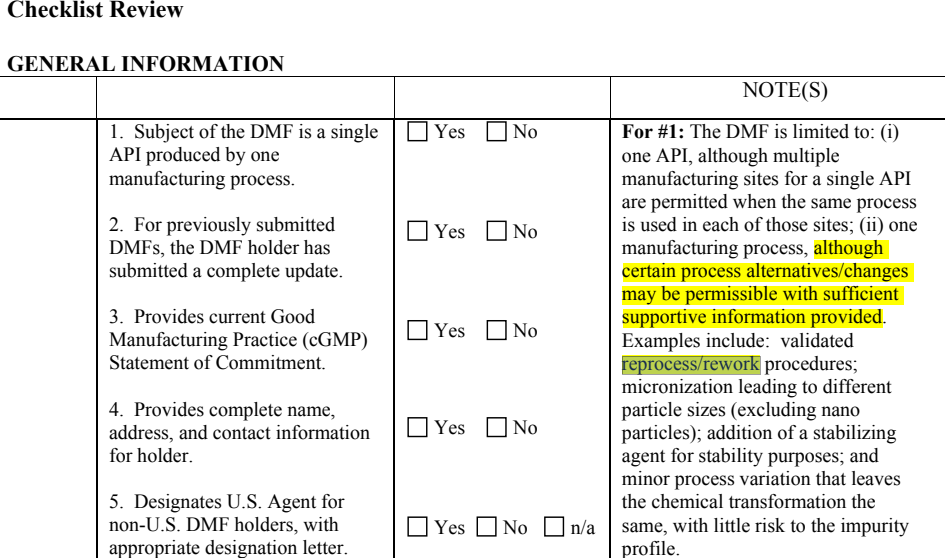
1) US方向:根据CA评估要求,可行,但操作空间有限,
前提条件:工艺路线相同,工艺差异较小,对杂质概况影响风险较小。
主要实现方式:精制工艺增加替代工艺(采用相同的合成路线,采用不同的精制溶剂或精制工艺略有差异)+
最低的产品质量标准;通过精制溶剂不同达到产品质量杂质含量水平差异的目的。
替代工艺不推荐开发最终精致的替代工艺,精烘包管理成本较普区高。
注:不同方向的申报,根据当前法规综合理解,除了粒径以及特殊剂型涉及的指标要求如微生物、内毒素等可根据使用目的设定,其他检查指标如含量与杂质接受标准应相同。
替代工艺存在的合理性主要通过对中间体或者最终产品质量(杂质和纯度)无明显影响进行论证。
US DMF CA评估具体要求如下:
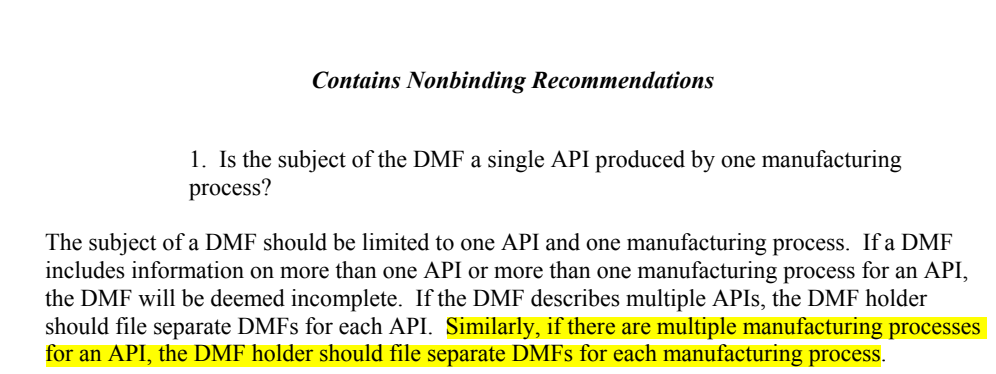
The DMF is limited to: (i) API produced
by one API, although multiple manufacturing sites for a single API are
permitted when the same process is used in each of those sites; (ii) one
manufacturing process, although certain
process alternatives/changes may be permissible with sufficient supportive
information provided. Examples include: validated reprocess/rework
procedures; micronization leading to different particle sizes (excluding nano
particles); addition of a stabilizing agent for stability purposes; and minor process variation that leaves the
chemical transformation same, with little risk to the impurity profile.
A separate DMF should be filed for:
different salt form; different synthetic route; and significant process variation
resulting in a different impurity profile and requiring a different control
strategy.
2) EU:根据Guidelineon the chemistry of active substances,可提供替代工艺,合理性应论证,应提供充分证明,最终产品质量保持不变。
因此,要在同一个ASMF中涵盖不同工艺,满足不同质量标准,也是应该通过提交替代工艺+最低产品质量标准实现。
指南描述如下:
Alternative processes
Alternative processes should be explained and
described with the same level of detail as the primary process. The process
description should fully define the method of synthesis. However, if
alternative steps or solvents are proposed they should be justified by providing sufficient evidence that the final quality
of the material (i.e. active substance or isolated intermediate) obtained
remains unchanged if the submission of data is via a CEP and/or an
ASMF.
Regarding
new active substances, if differences in impurity profiles are encountered,
they should be analysed with validated methods and shown to be toxicologically
acceptable.
3) EDQM:根据公开文件Contentof the dossier for chemical purity and microbiological quality要求,无本质差异的工艺才允许有替代工艺在同一份申报文件中。
指南要求如下:
Whatever
type of manufacturing process is used, alternatives within the same dossier are only allowed if not substantially
different. Even if the quality of
late stage key intermediates and final substance from the alternative process
are not affected in terms of specification and impurity content but the
processes are substantially different they cannot be accepted in the same
application. A separate CEP application covering the same substance with
the difference(s) explained in a sub-title may need to be submitted for each
alternative process.
4) CN方向:根据化学原料药、药用辅料及药包材与药品制剂关联审评审批管理规定(征求意见稿),个人理解法规不允许;在生产工艺原理相同的情况下,择优登记;原理不用,需要不同登记号。
在国家局2019年年底培训课件中也提到,
征求意见稿要求描述如下:
“登记号管理原则:同一企业在同一生产场地生产的同一原辅包产品,生产工艺和质量标准相同的,应按照同一登记号登记。
采用不同原理的生产工艺(如发酵、合成、半合成等)生产的同种原料药可按照不同登记号登记,采用相同原理的生产工艺生产的同种原料药应选择最优工艺进行登记。”
【主要参考法规或指南】
1、
2、
3、
4、化学原料药、药用辅料及药包材与药品制剂关联审评审批管理规定(征求意见稿)
不清楚你说的这个DMF是申报哪个市场, US, EU, 加拿大HC, 还是国内,也不清楚不同工艺指的是工艺上一些细微的参数的差别还是说合成路线不同。从问题来看应该比较大的工艺差别,而且导致了质量标准的不同。
这种情况下的话,从目前找到的法规来看,, US, EU, CA, 还是国内一般情况下都不允许一个DMF包括不同工艺而且不同的质量标准。
以下为查找的各国DMF指南中的说明,请参照理解。
依据:
国内:关于上线新版原辅包登记系统的通知 中有指出过:原则上,同一企业在同一生产场地生产的同一产品,生产工艺和质量标准相同的,应按照同一登记号登记;给药途径不同且质量标准存在差异的,应按照不同登记号登记,申请人可通过在产品名称后添加括号标注其给药途径的方式加以区分,如:利奈唑胺(供注射用)。
还有2017年发布的《原料药、药用辅料及药包材与药品制剂共同审评审批管理办法(征求意见稿)》第十六条:同一原料药生产企业供不同给药途径制剂使用且质量存在差别的同一原料药,应当按不同登记号登记;给药途径相同、生产工艺相近,仅晶型、粒径等质控要求不同的原料药,应当在同一登记号下对不同工艺、晶型、粒径进行分类并编号。
CDE 常见问题解答中也有相关问答:

US FDA:DMF 完整性评估指南中,明确 A separate DMF should be filed for: different salt form; different synthetic route; and significant process variation resulting in a different impurity profile and requiring a different control strategy.
CEP: CEP 变更指南 中指出:
In the following special cases a request for revision cannot be submitted but a separate CEP application should be made:
• Change to the manufacturing process resulting in
- Different polymorphic forms
- Introduction of a new substantially different route of synthesis (even when the
impurity profile of the final substance is equivalent)
EU ASMF: 原料药主文件问答
13. Can one ASMF holder have more than one ASMF for the same active substance but with different synthetic routes, specification, grade, etc.?
An ASMF holder may have more than one ASMF of a same active substance provided that the active substances have, inter alia, substantially different routes of synthesis, which result in changes to important quality characteristics of the active substance or different specifications. In this case, each ASMF should be assigned a different reference number by the ASMF holder; consequently, each ASMF will also require a separate EU/ASMF reference number. Slightly different routes of synthesis that do not result in changes to important quality characteristics should be incorporated into a single ASMF. If the ASMF holder is in doubt, they should contact an appropriate Competent Authority for guidance prior to submission of the ASMF.
加拿大HC:加拿大DMF指南
In some cases, a new Type I MF registration is required. The examples below indicate the criteria for new MF registrations:
- different active substance
- different salt of an active substance
- different complex of an active substance
- different co-crystal of an active substance
- different solvate or hydrate form of an active substance
- different isomer or mixture of isomers of an active substance
- racemate of an optically pure active substance
- optically pure enantiomer of a racemic active substance
- enantiomer of an active substance
- introduction of a new substantially different route of synthesis (i.e. resulting in a different specification for the active substance)
- different polymorphic forms (resulting in substantially different physicochemical and/or pharmacokinetic properties)
- any other change to the active substance that results in substantially different physicochemical and/or pharmacokinetic properties
- sterile grade of a non-sterile active substance
- non-sterile grade of a sterile active substance
- change/addition of raw materials of different animal origin (only where there is a substantial change in the safety of the active substance)
这{{threadTextType}}正{{isAdminText}}
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因: