EDQM实用工具knowledge database简介
Ø 工具介绍:Knowledge is a searchable database of information on a given substance or general method of analysis. It also contains information such as the revision history of monographs; chromatograms in PDF format; links to the reference standard catalogue number; trade names of some reagents, such as chromatography columns and biological kits; and access to the list of Certificates of Suitability (CEPs) of the European Pharmacopoeia monographs that have been granted for each substance.
知识数据库是关于药品或一般分析方法的可搜索信息的数据库,它包含个论的修订历史信息、PDF格式的色谱图、标准品信息、 某些试剂的商品名称,如色谱柱和生物试剂盒,以及获取已授予药品的CEP证书清单。
Ø 工具位置:登录EDQM官网,在界面下方的Useful information中找到database,点击后会有不同的database选项,选择knowledge database, 选择search,具体网址如下https://extranet.edqm.eu/publications/recherches_sw.shtml
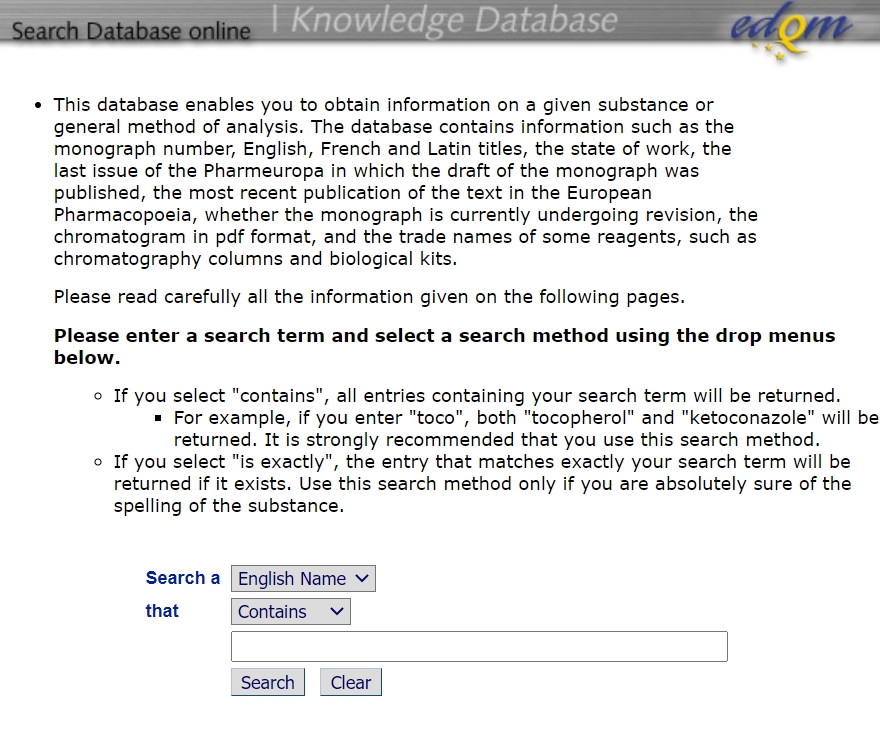
进入工具后的界面截图如下,页面上列出了该数据库的功能以及检索方式:
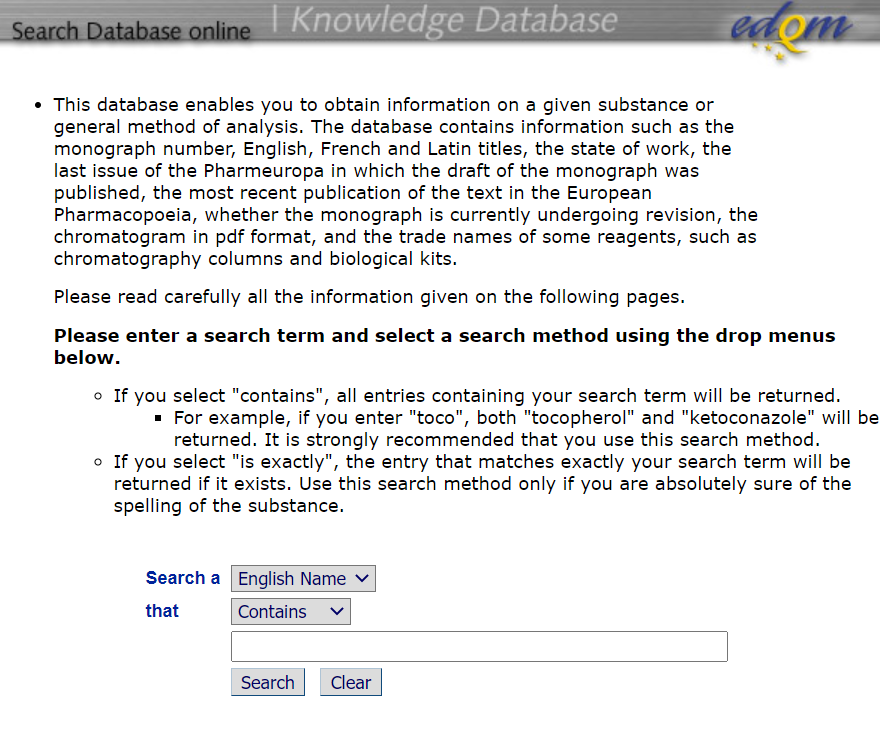
Ø 工具使用:如上截图的下侧搜索栏可以输入要检索的具体药品或者分析方法号的信息(名称或者章节号),支持精确查找和粗略查找两种方式,EDQM建议使用“contains”的选项进行检索,因为如果选择精确查找需要准确的输入药品的名称。
If you select "contains", all entries containing your search term will be returned.
- For example, if you enter "toco", both "tocopherol" and "ketoconazole" will be returned. It is strongly recommended that you use this search method.
- If you select "is exactly", the entry that matches exactly your search term will be returned if it exists. Use this search method only if you are absolutely sure of the spelling of the substance.
Ø 示例一:substance
我们以亚胺培南为例子进行检索,准确的英文名字imipenem monohydrate,章节号1226,通过输入章节号或者英文名字均可进行检索,我们选择contains,检索后界面如下,
点击1226链接进入:

在这个检索的页面,我们可以看到该章节目前属于在用状态,章节号01226,该章节是23.3药典论坛收录,在10.0版本发布。Chromatogram项下,点击Available,可以看到有关物质标准的典型色谱图如下

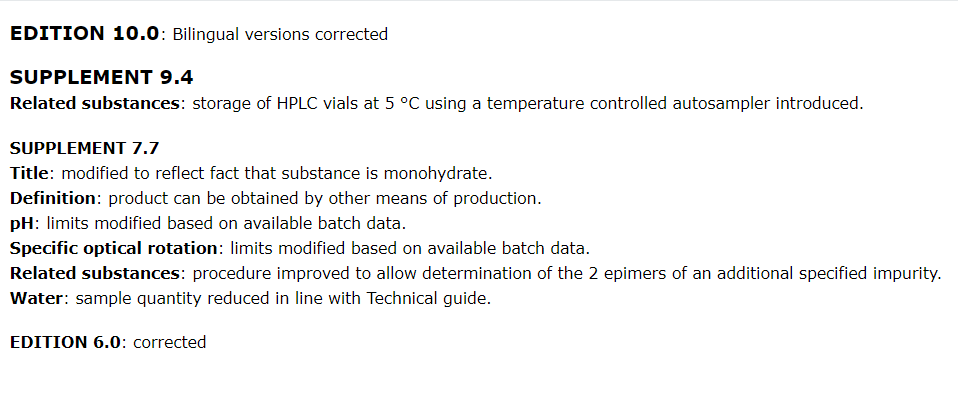
点击View history,可以看到该章节的药典修订历史,该章节的历史变化清晰明了。
 另外我们还可以看到标准品的信息、色谱柱的信息、试剂的信息,这些信息对于用户都具有较强的指导性和参考性。
另外我们还可以看到标准品的信息、色谱柱的信息、试剂的信息,这些信息对于用户都具有较强的指导性和参考性。
Knowledge database中对于各选项的详细解释如下,供参考。
- Status :“in use” the monograph is published in the European Pharmacopoeia, “elaboration” monograph is under elaboration and has not yet been published
- Monograph number : the unique number allocated to a monograph or a general method as soon as it is authorised for elaboration. This number never changes, contrary to the titles and should be used as a unique and unambiguous reference to a text of the European Pharmacopoeia.
- English, French and Latin names : these are the monograph or general methods titles as they are currently approved by the European Pharmacopoeia Commission. They may differ from the titles currently published in the Pharmacopoeia, especially for monographs currently at SOW 2 (see below). In case of any doubt, please refer to the monograph number.
- Published in Supplement : starting from 6.0, the first publication in the European Pharmacopoeia of the most recent version of the text in terms of technical content (i.e. no technical revision or correction has been made since this publication, but editorial modifications may have been made) has a first digit which is the edition number and the second which is the supplement number (0 represents the main volume of the edition in question).
- Work in progress : the boxes in light blue indicate whether a monograph is currently being elaborated or undergoing revision (Technical revision or Minor revision) and provides relevant information to the work on-going: current State of work and Pharmeuropa issue. Under “Description” if it is a revision, information on the test(s) revised and/or the reason for revision are provided. If you are interested in participating in the revision work and if the state of work is 0 or 1 please contact us through the Helpdesk so that you can be involved in the elaboration/revision process. We welcome your contributions to the work of the European Pharmacopoeia.
- State of work : the figures are to be interpreted as follows :
- 0 – The monograph has been authorised but work has not started yet
- 1 - Draft work has started
- 2 - Pharmeuropa the monograph has been authorised for publication in Pharmeuropa (see below)
- 3 - COM the monograph has been submitted for adoption to the European Pharmacopoeia Commission
- 4 - DEF the monograph has been adopted
Please note that texts which are published in Pharmeuropa (step 2 - public enquiry) can be consulted for free by registering on Pharmeuropa online , the procedure on how to comment is available here The supply of individual copies of our documents by fax, e-mail or otherwise, whatever their state of work, is not possible.
- Pharmeuropa : the issue of Pharmeuropa into which the draft of the monograph was published.
- Chromatogram :
- If a hyperlink appears in the adjacent cell, a type chromatogram is available for download. It should be stressed that such chromatograms do not constitute a mandatory part of the corresponding monograph and are provided for information only. They do not necessarily include all impurities mentioned in the monograph, are not representative for all impurity profiles of the substance and are provided solely for the convenience of the user.
- Please note that chromatograms supplied with some reference standards are available only if they are mentioned in the related monograph. They are sent to users on an official leaflet with the standards and are also available for download from the online CRS/BRP catalogue.
- Additional information : if a hyperlink appears in the adjacent cell, some information such as the background on the elaboration of the monograph is available. It should be stressed that such information is provided solely for the convenience of the user and does not constitute a mandatory part of the corresponding monograph.
- History : contains information concerning certain technical modifications to some revised/corrected texts published since Ph.Eur. 5.0. This information complements the modifications indicated by lines in the margin in the supplements and is not necessarily exhaustive.
- Interchangeable (ICH_Q4B) : the section reflects the status of the text with regard to the work of:
- The Pharmacopoeia Discussion Group (PDG), a joint collaboration between the United States Pharmacopeia, the Japanese Pharmacopeia and the European Pharmacopoeia.
- The International Conference on Harmonisation (ICH) Quality Guideline on Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH (Q4B).
- Pharmacopoeial harmonisation : further information can be found in chapter 5.8 (Pharmacopoeial Harmonisation) of the European Pharmacopoeia. Details about the status of individual texts and agreements reached on them by the three pharmacopoeias are shown in the tables and documents available in EDQM website.
- Reference standards : this is a list of the reference standard(s) that have to be used to carry out the tests in the monograph. For more information, please refer to the most up-to-date version of the monograph as published in the European Pharmacopoeia. This is an excerpt from our online CRS catalogue.
- Practical information :
- Certain tests require the use of commercially available reagents and/or chromatographic stationary phases, which cannot be described with the required accuracy in the European Pharmacopoeia. This page provides the trade name(s) of the reagent(s) and/or chromatographic stationary phase(s) that were found to be suitable when the monograph was being developed. This information is provided solely for the convenience of the users of the Pharmacopoeia. It does not imply that these reagents/stationary phases or their suppliers are endorsed or recommended by the European Pharmacopoeia Commission or the Council of Europe, in preference to others of a similar nature which are not mentioned. The analyst is also to be aware of the fact that some reagents/stationary phases can show significant batch to batch variations for which the EDQM cannot be held responsible.
- Also, if a hyperlink appears in the adjacent cell, some information is available about one or more of the tests in the monograph. This information consists of technical advice facilitating the achievement of the tests. It should be stressed that such information is provided solely for the convenience of the user and does not constitute a mandatory part of the corresponding monograph.
- CEP : if certificate(s) of suitability have been granted for the substance in question, the list of certificates is shown. This is an excerpt from the online List of CEP.
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:
