Note:文中法规和指南的英文部分为法规和指南原文,中文部分为作者解读和翻译,供参考。
1. 目的:实验室数据的第二人复核指分析员完成检测后,第二人对于检测结果的准确性的复核,本文结合国内外GMP法规、指南及数据完整性对于数据复核的相关要求,探讨实验室那些数据需要第二人的复核。
2. 术语:在展开讨论前,我们先了解下法规指南中关于审核和复核的相关术语:
Review 审核
Double check 再次确认
Four-eyes principle (a contemporaneous reading by a second analyst) 四眼原则
Independent verification 独立复核
A contemporaneous enumeration 同步计数
A contemporaneous verification 同步复核
Review need to be performed in real-time 实时审核
3. 法规和指南规定(第二人复核)
3.1 Medicines & Healthcare products Regulatory Agency (MHRA) ‘GXP’ Data Integrity Guidance and Definitions
Where the data obtained requires manual observation to record (for example results of a manual titration, visual interpretation of environmental monitoring plates) the process should be risk assessed and depending on the criticality, justify if a second contemporaneous verification check is required or investigate if the result could be captured by an alternate means.
对于人工观察并记录的数据,例如手工滴定,环境监测平皿计数,应基于风险及数据的关键程度,论述第二人的同步确认是否需要或者检测的结果用其他可替代的途径获取。
3.2 PICS Good Practices for Data Management and Integrity in Regulated GMP-GDP Environments 202107
Additional controls should be considered when critical test interpretations are made by a single individual (e.g. recording of microbial colonies on agar plates). A secondary review may be required in accordance with risk management principles. In some cases this review may need to be performed in real-time. Suitable electronic means of verifying critical data may be an acceptable alternative, e.g. taking photograph images of the data for retention.
当关键检测是由单人进行解读时(例如,记录琼脂平皿上的微生物菌落),应考虑额外的控制。根据风险管理原则,可能需要进行二次审核。在某些情况下,这种审核可能需要实时进行。可以接受采用合适的电子手段核实关键数据作为替代方法,例如:对数据拍照留存。
3.3 USP 1117 MICROBIOLOGICAL BEST LABORATORY PRACTICES
An important data integrity threat with microbiological testing resides with falsification of data and intentional omission of testing results. To control this risk, the company culture and ethical standards are essential as well as the application of a rigorous quality management system.
微生物检测的一个重要的数据完整性威胁在于伪造数据和故意遗漏检测结果。为了控制这种风险,公司文化和道德标准以及严格的质量管理体系的应用是必不可少的。
For the compendia sterility test that combines criticality of the test and higher risk of misinterpretation of results, it is now a standard practice to have a second analyst perform a contemporaneous evaluation of the sample (in test media) for microbial growth. Nonetheless, applying uncritically a contemporaneous reading by a second analyst (four-eyes principle) for all samples and microbiological tests is not recommended. Precision in counts may vary from one analyst to another (even if they are trained and qualified) as colonies may overlap, swarm over media, etc., allowing for misinterpretation. Microbiology is a “logarithmic science”; sample size is statistically weak and testing procedures have inherent variability. By tolerating no differences in counts, a high number of non-critical deviations will be generated, thus consuming resources unreasonably. As an alternative to a contemporaneous enumeration, a contemporaneous verification by a second person that the testing activity is performed correctly may be executed for higher risk tests. A second person could verify, for instance, if the reading of results is correctly executed according to the procedure, if the result on the Petri plate is correctly transcribed onto the GMP recording sheet (i.e. if growth is observed this is captured in the GMP sheet), and if the description of the sample corresponds to the description on the GMP recording sheet. An assessment of the risk due to a misinterpreted result and its impact on patient safety is performed to determine the high risk test outlined above.
考虑到检测项目的关键性和结果易出现差错的无菌检测,现在的通用的做法是让第二名分析人员对样品(培养基中)进行微生物生长的同时观察。尽管如此,不建议对所有样品和微生物测试不加批判地应用由第二个分析人员(四眼原则)同时进行读数。计数的精度可能因分析人员而异(即使他们受过训练并有资格),因为菌落可能重叠,聚集在培养基上,等等,允许误解。微生物学是一门“对数科学”;样本大小在统计上是微弱的,测试程序具有内在的可变性。如果不允许计数上的差异,就会产生大量的非关键偏差,从而不合理地消耗资源。作为同时枚举的一种替代方法,由第二个人同时验证测试活动是否正确执行,可以用于更高风险的测试执行验证。第二个人可以确认,例如,结果的读取是否按照程序正确执行,培养皿上的结果是否正确转录到GMP记录页上(即,如果观察到生长,则在GMP记录页上记录),并且样品的描述是否与GMP记录页上的描述相对应。对错误解释结果的风险及其对患者安全的影响进行评估,以确定上述高风险测试。
3.4 WHO ECSPP TRS 1033 Annex 4 Guideline on data integrity 202103
Example 3: Data entry
Data entry includes for example sample receiving registration, sample analysis result recording, logbook entries, registers, batch manufacturing record entries and information in case report forms. The recording of source data on paper records should be done using indelible ink, in a way that is complete, accurate, traceable, attributable and free from errors. Direct entry into electronic records should be done by responsible and appropriately trained individuals. Entries should be traceable to an individual (in electronic records, thus having an individual user access) and traceable to the date (and time, where relevant). Where appropriate, the entry should be verified by a second person or entered through technical means such as the scanning of bar-codes, where possible, for the intended use of these data. Additional controls may include the locking of critical data entries after the data are verified and a review of audit trails for critical data to detect if they have been altered. The manual entry of data from a paper record into a computerized system should be traceable to the paper records used which are kept as original data.
数据录入包括如下示例,例如样品接收登记、样品分析结果记录、日志录入、登记、批生产记录录入以及病例报告表中的信息。在纸质记录上记录源数据时,应使用不可擦除的墨水,且以完整、准确、可追溯、可归属且没有错误的方式记录。应由负责任的,经过适当培训的人员直接录入电子记录。录入应可追溯到个人(在电子记录中,单个用户访问),并且可追溯到日期(和时间,如相关)。在适用情况下,录入应由第二人核实,或尽可能针对这些数据预期用途通过技术手段录入,例如条形码扫描。其他控制可能包括:在数据核对后锁定关键数据录入,以及审查关键数据的审计追踪以检测数据是否被更改过。经数据从纸质记录手动录入到计算机化系统应该可以追溯到所使用的作为原始数据保存的纸质记录。
3.5 EU gmp Annex 11: Computerized Systems
6. Accuracy Checks
For critical data entered manually, there should be an additional check on the accuracy of the data. This check may be done by a second operator or by validated electronic means. The criticality and the potential consequences
关键数据的手动输入应由第二个操作员或经验证的计算机化方法复核。
3.6 PICS Good Practices for Data Management and Integrity in Regulated GMP-GDP Environments 202107
9.7 Data capture/entry for computerized systems.
- All manual data entries of critical data should be verified, either by a second operator, or by a validated computerized means.
- 所有关键数据的手动输入应由第二个操作员或经验证的计算机化方法复核。
3.7 对比分析:
l 对于人工观察并记录的实验室检测数据(例如手动滴定、平皿计数等),MHRA和PICS数据完整性指南中均要求要基于风险和数据的关键程度,必要时第二人同步复核或采取可替代的数据获取方式,
l USP 1117中规定,微生物检测的一个重要的数据完整性威胁在于伪造数据和故意遗漏检测结果,考虑到检测项目的关键性和结果易出现差错的无菌检测,现在的通用的做法是让第二名分析人员对样品(培养基中)进行微生物生长的同时观察。尽管如此,考虑到微生物检测项目的特殊性不建议对所有样品和微生物测试不加批判地应用由第二个分析人员(四眼原则)同时进行读数。
l 对于人工录入的关键数据需要第二个操作员或经验证的计算机化方法复核,EU GMP Annex 11 和PICS及WHO数据完整性指南中均有相关规定。
思考:综合上述分析,应基于风险和数据的关键程度来决定是否需要第二人复核,如何进行数据关键程度的风险评估。
4. 实验室关键数据的评估
4.1 WHO Guidance on good data and record management practices
Critical GXP:data with a direct impact on patient safety or product quality
关键数据:数据直接影响到病人的安全或最终产品的质量。
4.2 Medicines & Healthcare products Regulatory Agency (MHRA)
‘GXP’ Data Integrity Guidance and Definitions
4. Establishing data criticality and inherent integrity risk
4.1 Data has varying importance to quality, safety and efficacy decisions. Data criticality may be determined by considering how the data is used to influence the decisions made.
数据关键性及数据完整性风险
数据对于药品质量、安全及有效性的影响存在差异,数据的关键程度可以通过对于相关决策的影响程度来判定。
4.3 PICS GOOD PRACTICES FOR DATA MANAGEMENT AND INTEGRITY IN REGULATED GMP/GDP ENVIRONMENTS/ EU GMP DT Q&As
5.4 Data criticality
5.4.1 The decision that data influences may differ in importance and the impact of the data to a decision may also vary. Points to consider regarding data criticality include:
Which decision does the data influence?
For example: when making a batch release decision, data which determines compliance with critical quality attributes isnormally of greater importance than warehouse cleaning records.
What is the impact of the data to product quality or safety?
For example: for an oral tablet, API assay data is of generally greater impact to product quality and safety than tablet friability data.
数据的关键性
数据对于决策的影响与数据的重要性有关,数据对于决策的影响也存在差异,数据关键性的考虑点如下:
数据影响到什么决策?
例如:放行决策,影响到关键质量属性的测试要比仓库清洁记录重要。
对于产品质量和安全性的影响
例如:口服片剂,API含量检测数据要比片剂的松脆度关键。
Additional controls should be considered when critical test interpretations are made by a single individual (e.g. recording of microbial colonies on agar plates). A secondary review may be required in accordance with risk management principles. In some cases, this review may need to be performed in real-time.
4.4 EMA guidance on good manufacturing practice and good distribution practice questions and answers
How can data criticality be assessed?
The decision that data influences may differ in importance and the impact of the data to a decision may also vary. Points to consider regarding data criticality include:
Which decision does the data influence?
For example: when making a batch release decision, data which determines compliance with critical quality attributes isnormally of greater importance than warehouse cleaning records.
What is the impact of the data to product quality or safety?
For example: for an oral tablet, API assay data is of generally greater impact to product quality and safety than tablet dimensions data.
数据的关键性
数据对于决策的影响与数据的重要性有关,数据对于决策的影响也存在差异,数据关键性的考虑点如下:
数据影响到什么决策?
例如:放行决策,影响到关键质量属性的测试要比仓库清洁记录重要。
对于产品质量和安全性的影响
例如:口服片剂,API含量检测数据要比片剂的尺寸关键。
Additional controls should be considered when critical test interpretations are made by a single individual (e.g. recording of microbial colonies on agar plates). A secondary review may be required in accordance with risk management principles. In some cases, this review may need to be performed in real-time.
4.5 APIC Practical risk-based guide for managing Data Integrity
Data severity assessment: within CGxP data, different levels of severity can be defined as a function of its use. Typically, this is linked to the stage of manufacturing following the principle of increasing CGxP outlined in ICH Q7. Alternatively, other factors such as impact on final product quality can be taken into account to further differentiate between severity categories.
数据关键程度的评估应基于生产的步骤及对于最终产品质量的影响
4.6 PDA 80 Data integrity management system for pharmaceutical laboratories
Data integrity controls should be based on quality risk management principles such as ICH Q9, that is, the level of controls, verification, and oversight should be commensurate with the criticality of the data to patient safety and with the risk to data accuracy, completeness, fabrication or falsification.
数据完整性控制应基于质量风险管理原则,如ICH Q9,即控制、复核和监督的水平应与数据对患者安全的重要性以及数据准确性、完整性、伪造或伪造的风险相一致。
A risk matrix can be established using criticality of the test and maturity of the quality system and use of risk reducing technology. Data criticality can be established based on whether the test is for a critical quality attribute (CQA), such as a sterility test; a critical process control (CPC), such as an environmental monitoring test; or an in process control or other test, such as environmental monitoring for nonsterile products. Figure 7.21 shows a risk matrix applied to data integrity. In this example, the matrix is used to establish which microbiological tests require second person verification prior to approving test results.
结合检验的关键性、质量体系的成熟度和降低风险的技术,可以建立风险矩阵。可以根据测试是否为关键质量属性(CQA)(如无菌检查)来确定数据的关键性,关键工艺控制(CPC),如环境监测试验;或者过程控制/其他测试,如非无菌产品的环境监测。下图显示了应用于数据完整性的风险矩阵。在本例中,矩阵用于确定在批准测试结果之前,哪些微生物测试需要第二人复核。
微生物实验室数据关键程度矩阵图:
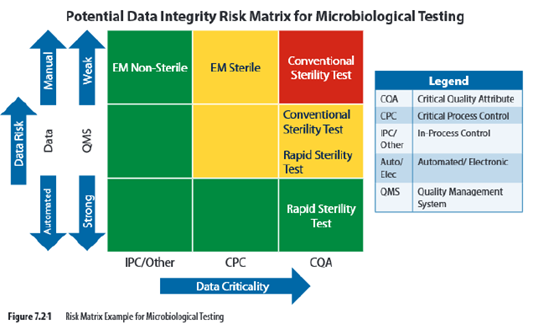
4.7 对比分析:
数据的关键程度应基于数据对于产品放行决策的影响和数据对于产品质量和病人用药的风险。
5. 总结:
5.1 QC内部需要人工观察并记录的相关数据不同于电子数据或打印输出数据,此类数据的追溯性相对较弱,出现人为差错后的可检测手段较电子数据或打印输出数据相对较少,结合相关数据完整性指南的要求,对于QC内部需要人工观察并手动记录的数据,应结合影响产品放行决策和对病人用药的安全性的影响进行数据关键性的评估,基于数据关键性及其风险,考虑进行第二人复核来降低风险。
5.2 同时对于关键数据的第二人复核的“同步/实时”,是否有时间限要求?应基于数据的可追溯性,选择适当的策略进行:
-数据承载的载体后续不可得,例如手工滴定分析,应做到同步并及时的结果复核;
-数据承载的载体可得但随时间的推移,检测结果会发生变化,例如溶液颜色,应做到同步并及时的结果复核;
-数据承载的载体可得可追溯,例如无菌检测,应在数据承载的载体(培养基)销毁前完成复核,可参考USP1117微生物样品观察时限的要求,观察的上午或下午完成检测结果的复核;需要注意的是微生物的平皿计数测试,虽载体可得可追溯,但平皿观察环境及培养时间可能对于后续的微生物生长产生影响,建议及时进行复核。
-对于关键数据的人工录入的第二人复核,可采取上述的原则进行。
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:
