PUBLIC DOCUMENT
(LEVEL 1)
English only
PA/PH/CEP (13) 110, 3 R
Strasbourg, November 2021
Certification of suitability to the Monographs of the European Pharmacopoeia
Management of applications for new Certificates of Suitability, Requests for Revision
or Renewal of Certificates of Suitability and applications using the ‘sister files’
procedure
管理欧洲药典适宜性证书的新申请、修订或更新及使用“姊妹文件”程序的申请
Revision history of the document 文件修订历史
|
Revision N° |
Revision date 修订日期 |
Reason
原因 |
|
R3 |
November 2021 |
Update following implementation of new IT tool for the
handling of CEP applications (changes to EDQM timelines now expressed in working days (WD) 在实施新的IT工具后进行更新,以处理CEP应用程序(EDQM时间表现在变更以工作日表示) |
1. Introduction 简介
This document describes the process applied to manage the applications for new certificates of suitability (CEPs), applications for revisions/renewals of existing certificates of suitability and via the ‘sister files’ procedure .
本文件介绍管理新适宜性证书、修订/更新现有适宜性证书的申请,以及通过“姊妹文件”程序的申请。
This document should be read in conjunction with: 本文件应与下列文件一起阅读:
- RESOLUTION AP-CSP (07) 1, Certification of suitability to the monographs of
the European Pharmacopoeia, which describes the procedure to grant and revise CEPs. 决议AP-CSP(07)
1,适用于欧洲药典各论的认证,描述了批准和修订CEPs的程序。
- EDQM “Guideline on Requirements on Revision/Renewal of Certificates of Suitability to the European Pharmacopoeia monographs” (PA/PH/CEP (04) 2), which describes the categorisation of requests to revise CEPs and the conditions to be fulfilled as well as the documentation to be submitted for each request for revision. EDQM《欧洲药典各论适宜性证书修订/更新要求指南》(PA/PH/CEP(04) 2),描述了修订CEPs请求的分类、需要满足的条件以及每次修订请求需要提交的文件。
- EDQM “Guidance on applications for sister files” (PA/PH/CEP (09) 141), which describes the conditions to be fulfilled as well as the documentation to be submitted for applications using the sister files procedure. EDQM“姊妹文件申请指南”(PA/PH/CEP(09)
141),描述了使用姊妹文件程序申请所需满足的条件以及需要提交的文件。
2.Applications for new CEPs, revision or renewal of CEPs or applications via the ‘sister files’ procedure - excluding notifications and transfer of holdership申请新的CEPs、修订或更新CEPs或通过“姊妹文件”程序申请-不包括通知和转让持有人
2.1Overview概述
EDQM acknowledges reception of applications and responses to requests for information submitted with respect to applications within 5 working days of receipt . An acknowledgement of receipt is sent, which specifies that the clock has started together with the deadline for the treatment of the request. EDQM在收到申请后的5个工作日内,确认收到申请及回应要求有关申请资料的要求。发送一个接收确认,指定计时已经开始,以及处理请求的最后期限。
As shown in annex 1, Process Flows, once the application is validated for evaluation and the clock has started, the assessment of applications is handled by: 如附件1流程流程所示,一旦应用程序验证并进行评估,计时启动,应用程序的评估由:
- if necessary, the evaluation of additional information submitted upon request from the EDQM, and如有必要,对EDQM要求提交的附加信息进行评估
- if necessary, the evaluation of another, last, package of additional information submitted upon a last request from the EDQM .
如有必要,对EDQM最后一次请求时提交的另一个最后的附加信息包进行评估。
The timings allowed for the EDQM and for the applicant at the different steps in the process are included in the table in annex 2 and these vary depending on the type of application (e.g. new application, type of revision etc.). 在申请程序的不同步骤,EDQM及申请人所允许的时间已列于附件2的表格内,这些时间视乎申请类别而定(例如新申请、修订类别等)。
Where the assessment is successful and the application is accepted as complete, a CEP (new or revised) is granted or for some revisions, a letter is sent to advise that the application has been accepted and the current CEP remains valid. The type of revision has an impact on whether the CEP is revised or not following acceptance of a revision as shown in annex 2 . 如评估成功,并接受完整的申请,则会获发一份新的或经修订的CEP,或在作出一些修订后,会发信通知申请已获接纳,而现行的CEP仍有效。如附件2所示,修订的类型会影响CEP是否在接受修订后进行修订。
Applications lacking sufficient information after evaluation of the applicant’s response to a second request for information from EDQM are definitively closed and the applicant is advised by letter. EDQM对申请人的第二次信息请求的回应进行评估后,如果申请人缺乏足够的信息,将最终关闭申请,并以信件的形式通知申请人。
For new applications and sister file applications, this means the application is rejected without a CEP being granted. For revision applications, this means that the request for revision is rejected and the current version of the CEP (without the requested changes) remains valid. For renewal applications this will have an impact on the validity of the existing CEP which may be suspended or withdrawn (see section 6) . 对于新申请和姊妹文件申请,这意味着申请在没有授予CEP的情况下被拒绝。对于修订应用程序,这意味着修订请求被拒绝,而CEP的当前版本(不包含所请求的变更)仍然有效。就更新申请而言,这将会影响现有的CEP的有效性,该CEP可能会被暂停或撤回(见第6条)。
2.2 Outcome of the assessment of the initial submission初次提交的评估结果
When the initial assessment of the application is complete there are 4 possible conclusions as described in below: 当完成对申请的初步评估后,可得出以下4个可能的结论:
2.2.1 The information provided is complete and the certificate can be granted
or the request for revision is accepted. 所提供的资料是完整的,可以发出证书或接受修订的要求。
A CEP (new, revised or renewed) is granted or the applicant is informed that the revision request has been accepted, see annex 2 . 申请人会获发一份新的、经修订或更新的CEP,或获通知修订申请已获接纳,见附件2。
2.2.2 The information provided is unsatisfactory and the certificate cannot be
granted or the request for revision/renewal accepted until the issues identified are resolved. 所提供的资料不令人满意,在发现的问题解决之前,不会发出证书或接受修订/更新的要求。
The EDQM sends a letter to the applicant requesting additional information in which there is a list of questions relating to the issues outstanding with the submission which prevent the request being accepted .
EDQM向申请人发送一封信,要求提供额外的信息,其中列出了与提交文件中未解决的问题有关的问题清单,这些问题阻止了请求被接受。
The applicant/holder has a defined time (see annex 2) to reply to this request and provide the information required. 申请人/持有人有一个规定的时间(见附件2)来答复此请求并提供所需的信息。
The applicant/holder should reply to this request and provide full information required by the deadline or the assessment may be stopped due to this failure to respond (see section 5). 申请人/持有人应在截止日期前回复此请求并提供所需的全部信息,否则评估可能会因未能回复而停止(见第5节)。
On reception of the response by EDQM, an e-mail confirming receipt of the data is sent to the applicant. The submitted information requires assessment and the deadline for the assessment of the response and the provision of a response to the applicant are as described in the e-mail and in annex 2 .
EDQM收到回复后,会向申请人发送确认收到数据的电子邮件。提交的信息需要评估,评估答复和向申请人提供答复的截止日期如电子邮件和附件2所述。
2.2.3 The information provided is in general satisfactory but clarification is
required on some issues before the certificate is granted所提供的资料一般来说是令人满意的,但在颁发证书前,有些问题需要澄清
This refers to the situation when there are some minor remaining point(s)/needs for clarification which have to be addressed . 这指的是还有一些需要澄清的次要问题需要解决的情况。
Following this conclusion, the EDQM sends a letter to the applicant requesting clarification of the issues outstanding with the submission which prevent the request being accepted . 在得出这一结论后,EDQM致函申请人,要求澄清该提交文件中未解决的问题,这些问题阻碍了该请求被接受。
The applicant/holder has a defined time (see annex 2) to reply to this request and provide the information required.
申请人/持有人有一个规定的时间(见附件2)来答复此请求并提供所需的信息。
The applicant/holder should reply to this request and provide full information required by the deadline or the assessment may be stopped due to a failure to respond (see section 5). 申请人/持有人应在截止日期前回复此要求,并提供所需的全部资料,否则评估可能因未能回复而终止(见第5节)。
On reception of the response by EDQM, an e-mail confirming receipt is sent to the applicant. The submitted information requires checking and the deadline for the checking of the response and the provision of a response to the applicant are as described in the e-mail and in annex 2 . EDQM收到回复后,会向申请人发送确认收到的电子邮件。提交的信息需要审核,审核答复和向申请人提供答复的截止日期如电子邮件和附件2所述。
2.2.4 The information provided is satisfactory but sections of module 3 require to
be updated to reflect the approved dossier before the certificate can be granted or the request for revision/renewal is accepted . 所提供的资料令人满意,但模块3的部分必须更新,以反映已获批准的档案,才可获发证书或接受修订/更新的要求。
This refers to the situation when there must be an update of the information provided in module 3 to reflect the approved data.
For example, where information provided in the module 1 response has not been incorporated into the appropriate section(s) of module 3. 这是指必须更新模块3中提供的信息以反映批准的数据的情况。例如,模块1中提供的信息没有被纳入模块3的适当部分。
Following this conclusion, the EDQM sends a letter to the applicant requesting update of the sections of module 3 to reflect the approved dossier. 在此结论之后,EDQM向申请人发出一封信,要求更新模块3的部分,以反映已批准的档案。
The applicant/holder has a defined time (see annex 2) to reply to this request and provide the information required. 申请人/持有人有一个规定的时间(见附件2)来答复此请求并提供所需的信息。
The applicant/holder should reply to this request and provide full information required by the deadline or the assessment may be stopped due to a failure to respond (see section 5). 申请人/持有人应在截止日期前回复此要求,并提供所需的全部资料,否则评估可能因未能回复而终止(见第5节)。
An e-mail confirming receipt is sent by EDQM and there is no assessment of the response received for a request to update a dossier. The module 3 is reviewed to ensure it has been updated as
required and if so, then the process to prepare the certificate or
to confirm acceptance of a request for revision begins. It is anticipated that the CEP or letter of approval will be issued within 23 working days of the receipt of the updated dossier. EDQM发送了一封确认收到的电子邮件,并且没有对更新档案请求收到的响应进行评估。对模块3进行审核,以确保它已按要求更新,如果是这样,则开始准备证书或确认接受修订请求的过程。预计在收到更新后的档案后23个工作日内,当局会发出CEP或批准信。
2.3 Outcome of the assessment of the response to the 1st request for
additional information. 对第一次额外信息请求响应的评估结果。
After validation of the response provided by the applicant to the 1st request for additional information, EDQM has a period of time to perform the
assessment and inform the applicant/holder of the conclusion of the assessment. The timings allowed are described in annex 2. 在申请人第一次要求提供额外信息的答复得到确认后,EDQM有一段时间进行评估,并将评估结果通知申请人/持证人。所允许的时间安排见附件2。
When the assessment of the response is complete there are 5 possible conclusions comprising the four conclusions described in 2.2.1 to 2.2.4 and the additional conclusion described in 2.3.1. 当反应评估完成时,有5个可能的结论,包括2.2.1至2.2.4中描述的4个结论和2.3.1中描述的附加结论。
2.3.1 Based on the information provided, the conclusion is that no certificate
can be granted or the request for revision is rejected with no further request for information . 根据提供的信息,结论是不能颁发证书,或者在没有进一步信息请求的情况下拒绝修改请求。
For an application for a new certificate (including those using the sister file procedure), the outstanding points are identified and the EDQM sends a letter to the applicant, informing them that the application is being closed and providing a list of the outstanding issues which prevent the granting of a certificate and have led to the rejection .对于新证书的申请(包括使用姐妹文件程序的申请),EDQM会识别未解决问题,并向申请人发送一封信,通知他们申请已经结束,并提供一份阻止颁发证书并导致拒绝颁发证书的未解决问题清单。
The application is definitely closed. However, the applicant may submit a new application for the same substance. It is expected that the outstanding issues from the assessment of the original closed dossier will be addressed in the new submission. 应用程序肯定是关闭的。但是,申请人可以对同一物质提出新的申请。预计在新的提交文件中将处理评估原来的封闭档案所遗留的未解决问题。
For requests for revision, the outstanding points are identified and the EDQM sends a letter to the applicant informing that the revision request is rejected and including a list of the issues outstanding which prevent the approval of the request for revision. This also means that the current version of the CEP (without the requested changes) remains valid. 对于修订请求,会找出未解决的问题,EDQM会致函申请人,通知其修订请求被拒绝,并附上妨碍批准修订请求的未解决问题清单。这也意味着CEP的当前版本(不包含所请求的更改)仍然有效。
The applicant may submit another revision request for the CEP dossier for the same changes. It is expected that the outstanding issues from the assessment of the original request for revision will be addressed in the new request.申请人可以就相同的变更向CEP档案提交另一份修订请求。预期在新的请求中将处理对原来的修订请求进行评估所产生的尚未解决的问题。
2.4 Outcome of the assessment of the response to the 2nd request for
additional information. 对第二次额外信息请求响应的评估结果。
After validation of the response provided by the applicant to the 2nd request for additional information, EDQM has a period of time to perform the
assessment and inform the
applicant/holder of the conclusion of the assessment. The time periods are described in annex 2 . 在申请人第二次要求提供额外资料的答复得到确认后,EDQM有一段时间进行评估,并将评估结果通知申请人/持有人。时间段载于附件2。
When the assessment of the response is complete there are only 3 possible conclusions: 当回复评估完成时,只有3个可能的结论:
2.4.1 The information provided is complete and the certificate can be granted or
the request for revision/renewal is accepted. 所提供的资料已完整,本署可发出证书,或接受有关修订/更新的要求。
A CEP is granted or the applicant/holder is informed that the revision has been accepted, and the current CEP remains valid (see annex 2) . 申请人/持证人会获发CEP,或获通知有关修订已获接纳,而现行的CEP仍有效(见附件2)。
2.4.2 Based on the information provided, the conclusion is that no certificate can
be granted or the request for revision is rejected. 根据提供的信息,结论是不能颁发证书,或者修改请求被拒绝。
For an application for a new certificate (including those using the sister file procedure), if there are outstanding points, the application is rejected with no further request for information. The EDQM sends a letter to the applicant informing them that the application is being closed and providing a list of the outstanding issues which prevent the granting of a certificate and have led to the closure. 对于新证书的申请(包括使用姊妹文件程序的申请),如果有未解决的问题,申请将被拒绝,并不再要求提供信息。EDQM向申请人发出一封信,通知他们申请已经终止,并提供了一份未解决的问题清单,这些问题阻止颁发证书,导致了终止申请。
The application is definitely closed. However, the applicant may submit a new application for the same substance. It is expected that the outstanding issues from the assessment of the original closed dossier will be addressed in the new submission. 应用程序肯定是关闭的。但是,申请人可以对同一物质提出新的申请。预计在新的提交文件中将处理评估原来的封闭档案所遗留的未决问题。
For requests for revision, the EDQM sends a letter to the applicant informing that the revision request is rejected and including a list of the issues outstanding which prevent the approval of the request for revision. If appropriate, the letter also includes a request to update the sections of module 3 to remove the information which has been rejected and therefore to reflect the approved dossier. This also means that the current version of the CEP (without the requested changes) remains valid. 对于修订请求,EDQM会致函申请人,通知其修订请求被拒绝,并附上妨碍批准修订请求的未解决问题清单。如果合适的话,这封信还包括更新模块3部分的请求,以删除已被拒绝的信息,从而反映已批准的档案。这也意味着CEP的当前版本(不包含所请求的更改)仍然有效。
The applicant may submit another revision request for the CEP dossier for the same changes. It is expected that the outstanding issues from the assessment of the original request for revision will be addressed in the new request.申请人可以就相同的变更向CEP档案提交另一份修订请求。预期在新的请求中将处理对原来的修订请求进行评估所产生的尚未解决的问题。
When a renewal application cannot be accepted then there may be an impact on the validity of the CEP, see section 6.
如果更新申请不被接受,则CEP的有效性可能会受到影响,见第6节。
2.4.3 The information provided is satisfactory but sections of module 3 require
to be updated to reflect the approved dossier before the certificate can be granted or the request for revision/renewal accepted . 所提供的资料是令人满意的,但模块3的部分必须在证书获发或修订/更新的要求被接受之前更新,以反映已获批准的档案。
This refers to the situation when there must be an update of the information provided in module 3 to reflect the approved data, for example, where information provided in the module 1 response has not been incorporated into the appropriate section of module 3. 这指的是模块3中提供的信息必须更新以反映批准的数据的情况,例如模块1答复中提供的信息没有被纳入模块3的适当部分。
Following this conclusion, the EDQM sends a letter to the applicant requesting update of the sections of module 3 to reflect the approved dossier. 在此结论之后,EDQM向申请人发出一封信,要求更新模块3的部分,以反映已批准的档案。
The applicant/holder has a defined time (see annex 2) to reply to this request and provide the information required. 申请人/持有人有一个规定的时间(见附件2)来答复此请求并提供所需的信息。
The applicant/holder should reply to this request and provide full information required by the deadline or the assessment may be stopped due to a failure to respond (see section 5). 申请人/持有人应在截止日期前回复此要求,并提供所需的全部资料,否则评估可能因未能回复而终止(见第5节)。
An e-mail confirming receipt is sent by EDQM and there is no assessment of the response received for a request to update a dossier. The module 3 is reviewed to ensure it has been updated as
required and if so, then the process to prepare the certificate or
to confirm acceptance of a request for revision begins. It is anticipated that the CEP or letter of approval will be issued within 23 working days of the receipt of the updated dossier. EDQM发送了一封确认收到的电子邮件,并且没有对更新档案请求收到的回复进行评估。对模块3进行审核,以确保它已按要求更新,如果是这样,则开始准备证书或确认接受修订请求的过程。预计在收到更新后的档案后23个工作日内,当局会发出CEP或批准信。
3. Management of Notifications 通知的管理
Immediate Notifications have to be reported immediately after implementation within the company whilst annual notifications may
be reported by way of a report compiling all the changes, which meet the conditions to be classified as annual notifications, which have been implemented within 12 months of the implementation of the first such change. 立即通知必须在公司内部实施后立即报告,而年度通知可以通过一份汇总所有变更的报告的方式报告,这些变更符合年度通知的条件,并已在首次变更实施后的12个月内实施。
The reporting of annual notifications is based on the implementation date of the changes and not the anniversary of the CEP application. 年度通知的报告是根据修订的实施日期,而非CEP申请的周年。
Notifications are either accepted or rejected (if the conditions for a notification are not met) . EDQM informs the applicant of the decision regarding the notification within 23 working days of its reception. If a notification is accepted, then either a revised certificate is granted or a letter is sent from EDQM to the holder advising that the notification has been found valid. Notifications which are accepted will result in revision of the CEP only where the information on the CEP requires to be changed . 接收或拒绝通知(如果通知的条件不满足)。教育局在收到通知后23个工作日内,将有关决定通知申请人。如果一份通知被接受,那么EDQM会颁发一份修改后的证书,或者给持证人发一封信,告知该通知是有效的。只有在CEP上的资料需要变更的情况下,获接纳的通知才会导致CEP的修订。
When a notification is rejected a letter is sent from EDQM to the holder advising that it has not been accepted and there is no possibility to submit additional information . 当通知被拒绝时,EDQM会向持有人发送一封信,通知其未被接受,并且不可能提交额外的信息。
Changes which have been rejected as notifications should be submitted again and depending on the reasons for rejection (which should be addressed in the new request) the type of revision submitted should be adapted (generally a minor revision by default) 作为通知被拒绝的变更应再次提交,并根据拒绝的原因(应在新请求中说明),提交的修订类型应调整(通常默认为小修订)
4. Transfer of holdership 持有人转移
A transfer of holdership is completed within 23 working days after receipt of a request, and a revised certificate is granted. 在收到申请后的23个工作日内完成转业手续,并获发经修订的证书。
If the request is incomplete at receipt, it is rejected without asking for any additional information and a letter of rejection is sent to the holder.如果在收据上的请求不完整,在没有要求任何额外信息的情况下被拒绝,并向持票人发出拒绝信。
5. Administrative closure of an application for a new CEP or a revision or renewal or a sister file due to lack of response within the required time因未能在规定时间内作出回应,而以行政方式终止新CEP或修订、更新或姊妹文件的申请
At each point in the assessment process, where a request for information is sent by the EDQM to the applicant/holder, the time by which the response should be received is clearly indicated in the letter. 在评审过程的每一阶段,当EDQM向申请人/持有人发出索取资料的要求时,应在信中清楚注明收到答复的时间。
For an application for a new certificate or a sister file, if no response is provided by the applicant within the time allocated then the dossier is closed without further notice and a letter is sent by EDQM to the applicant informing them
that the dossier has been closed. The
dossier is definitively closed. However, the applicant may submit a new
application for the same substance. It is expected that the outstanding issues from the assessment of the original closed dossier will be addressed in the new submission. 对于新证书或姊妹文件的申请,如果申请人在指定时间内没有作出回应,则档案会在不另行通知的情况下关闭,EDQM会向申请人发出信件,通知其档案已关闭,档案最终被关闭。但是,申请人可以对同一物质提出新的申请。预计在新的提交文件中将处理评估原来的关闭档案所遗留的未决问题。
For requests for revision, the EDQM sends a letter to the applicant informing them that the lack of response has prevented the approval of the request for revision, which is therefore closed . This also means that the current version of the CEP (without the requested changes) remains valid. There may also be a requirement to update the module 3 to remove the proposed changes (see 2.2.4). 对于修订请求,EDQM会给申请人发一封信,通知他们由于没有得到回复而无法批准修订请求,因此修订请求已被关闭。这也意味着CEP的当前版本(不包含所请求的变更)仍然有效。可能还需要更新模块3以删除提议的变更(见2.2.4)。
The applicant may submit another revision request for the certificate for the same changes. It is expected that the outstanding issues from the assessment of the original request for revision will be addressed in the new request. 申请人可就相同的变更,再次提出证书修订要求。预期在新的请求中将处理对原来的修订请求进行评估所产生的尚未解决的问题。
For requests for renewal, the
EDQM informs the applicant that the lack
of response has prevented the approval of the request for renewal. This will affect the validity of the current CEP (see section 6). 对于更新申请,EDQM会通知申请人,由于缺乏回复,更新申请无法获得批准。这将影响当前CEP的有效性(见第6节)。
6. Suspension or Withdrawal of a CEP following rejection of a revision or renewal在拒绝修订或更新后,暂停或撤回CEP
In specific situations of renewal of
a CEP or update of CEP application after a Ph. Eur . monograph revision, if the application is rejected because the CEP dossier is not in compliance with the current regulatory requirements, the validity of the CEP cannot be maintained . One example of when this may happen are when a monograph revision includes a new control/limit which has been added for safety considerations and where the substance covered by the CEP is not compliant with the requirement of the monograph. The outstanding points are identified and the EDQM sends a letter to the applicant informing them of all the issues outstanding which prevent the approval of the request . The holder should take the necessary actions to address the issues .在具体情况下,在欧洲药典修订后更新CEP或更新CEP申请,如果由于CEP档案不符合现行法规要求而拒绝申请,则CEP的有效性无法维持。可能发生这种情况的一个例子是,某一专论修订包括一个新的控制/限度,该控制/限度是出于安全考虑而添加的,而CEP所涵盖的物质不符合该专论的要求。未解决的问题被识别出来,EDQM会给申请人发一封信,通知他们所有未解决的问题,这些问题阻止了申请的批准。持证人应采取必要措施解决问题。
Where the holder of the CEP fails to respond to EDQM requests or is not able to produce a substance which is compliant with the monograph then the CEP may be suspended or withdrawn.如果CEP持有人未能对EDQM要求作出回应,或不能生产出符合各论的物质,则CEP可能会被暂停或撤销。
Similarly, if the holder fails to meet the requirements for the renewal of an application, the CEP will be considered as definitely expired and cannot be restored.同样,如果持有人未能符合更新申请的规定,该CEP将会被视为过期而不能恢复签发。
In such cases where the CEP is suspended or withdrawn by EDQM the procedure is described in “Suspension or Withdrawal of a Certificate of Suitability, Closure of an Application”, PA/PH/CEP (08) 17.在EDQM暂停或撤销CEP的情况下,程序在“暂停或撤销适宜性证书,关闭申请”,PA/PH/CEP(08) 17中描述。
7. Documents referenced:参考文件
Certification of suitability to the monographs of the European Pharmacopoeia欧洲药典各论的适宜性认证
Guideline on Requirements on Revision/Renewal of Certificates of Suitability.修订/更新适宜性证书的规定指南
Guidance on applications for sister files姊妹文件申请指南
Suspension or Withdrawal of a Certificate of Suitability, Closure of an Application暂停或撤销适宜性证书,关闭申请
Annex 1 –Process Flows 过程流程图
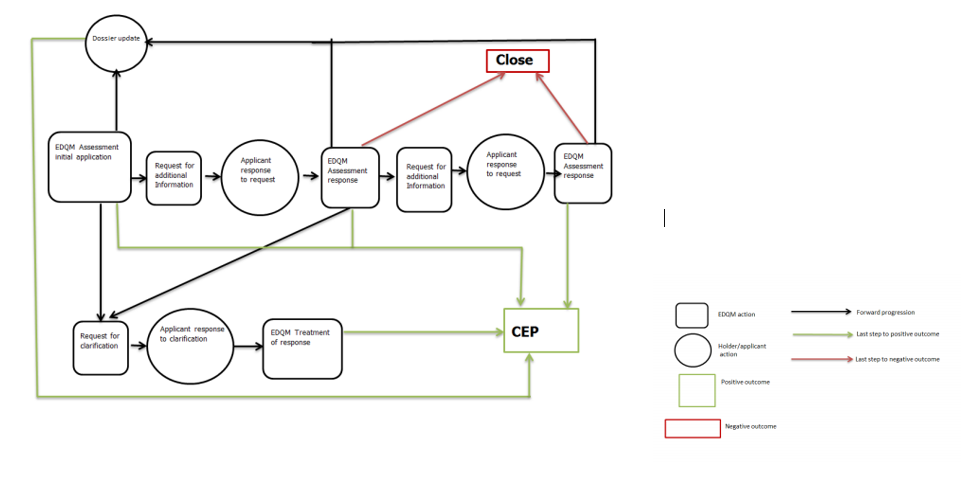
Applications for New CEP and sister file 新CEP和姊妹文件的申请
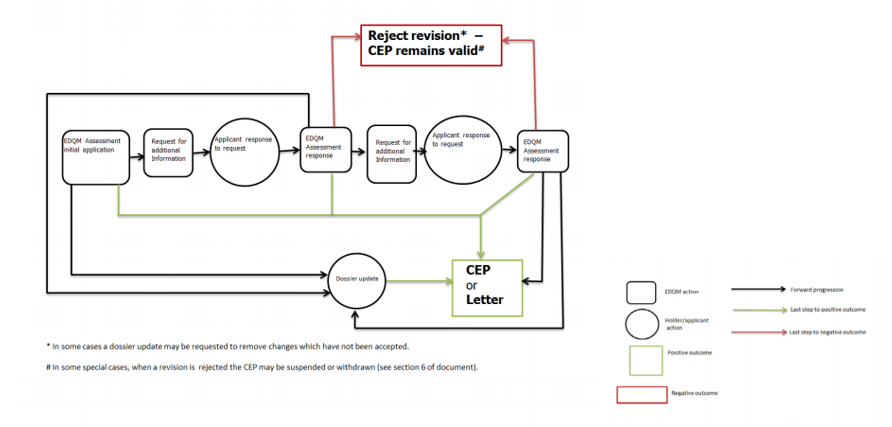
Applications for Revisions or Renewals 修订或更新的申请
Annex 2 – Timings and details of whether CEPs are revised/granted if application accepted
如申请获接纳,CEPs修订/批出的时间及详情
|
Type of application申请类型 |
EDQM Timelines for assessment of initial Application EDQM对初步申请的评估时间 |
Applicant Timeline to reply to first request for additional Information 申请者对首次额外信息要求的回复的时间 |
EDQM Timelines for assessment of reply to request for Information EDQM对额外信息要求的回复的评估时间 |
Applicant Timeline to reply to second request for additional Information 申请者对第二次额外信息要求的回复的时间 |
EDQM Timelines for assessment of reply to request for Information EDQM对额外信息要求的回复的评估时间 |
CEP revised if application accepted ? 如申请获接纳,CEP会否修订? |
|
New 新申请 |
115 WD ° |
180 CD* |
92 WD* |
90 CD * |
92 WD * |
New CEP issued 发布新CEP |
|
30 CD # |
23 WD # |
30 CD # |
23 WD # |
|||
|
Sister file 姊妹文件 |
46 WD |
30 CD + |
23 WD |
30 CD |
23 WD |
New CEP issued 发布新CEP |
|
Minor revision(s) 微小修订 |
23 WD |
30 CD |
23 WD |
30 CD |
23 WD |
Revised CEP issued only if information on CEP requires to be changed otherwise a letter accepting the revision is Issued 只有在CEP上的信息需要变更时,才会发出修订的CEP,否则会发布一封接受修订的信 |
|
Major revision 关键修订 |
46 WD |
30 CD |
23 WD (TSE or Herbal:46 WD ) |
30 CD |
23 WD (TSE or Herbal:46 WD ) |
Revised CEP issued 发布修订的CEP |
|
Monograph Revision 各论修订 |
69 WD |
30 CD |
23 WD (TSE or Herbal:46 WD ) |
30 CD |
23 WD (TSE or Herbal:46 WD ) |
Revised CEP issued only if information on CEP requires to be changed otherwise a letter accepting the revision is Issued 只有在CEP上的信息需要变更时,才会发出修订的CEP,否则会发布一封接受修订的信 |
|
Renewal 更新 |
69 WD |
30 CD |
23 WD (TSE or Herbal: 46 WD) |
30 CD |
23 WD (TSE or Herbal:46 WD ) |
Renewed CEP issued 发布更新的CEP |
* if the request from EDQM relates to significant information required to address the issues identified
# if the request from EDQM relates to clarification of minor issues or update of the dossier
° EDQM timelines are expressed in working days (WD): week-end, bank holidays and EDQM closures are not taken into account in the calculation + CD = Calendar days
*如果EDQM的要求涉及解决已确定问题所需的重要信息
#如果EDQM的要求涉及澄清小问题或更新档案
°EDQM时间线以工作日(WD)表示:周末、银行假期和EDQM关闭不计入计算
+ CD =日历天数
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:
