Why is Steam Quality Testing considered so important by SRA inspectors? What should a company wishing to be compliant with WHO/PICs/EU/MHRA cGMP be doing and why?
Ian Thrussell – GMP Consultant, Park Pharma Compliance Solutions and ex MHRA Expert Inspector, ex Head of Inspections WHO Prequalification Team
Introduction
During my foreign GMP inspections as a regulator and later as a consultant auditor, I frequently find a lack of knowledge of the importance of steam quality to sterilisation, the failure to perform appropriate risk-based steam testing in their management of sterilisers and SIP systems, how stem quality should be assessed and when, and particularly why it is important for the assurance of sterile operations.
This document seeks to bridge a gap in that knowledge and provide a summary of expectations from SRA inspectors that may arise during inspections. This introduction and a good understanding of the principles by production, engineering and QA staff should allow SMEs to be better prepared for inspection, but most of all better protect the patients receiving pharmaceutical products from the harm resulting from administration of a contaminated injection.
As I have personally been infused with a contaminated aseptically manufactured product during a hospital stay in Europe about 5 years ago, I can tell you this is not a pleasant experience!!!! Of course, in my case, the contamination causing my acute adverse reaction of pyrogenic shock, may not have been due to contaminated steam as the manufacture of any aseptically prepared product is a process with multiple risks and multiple serial control measures to protect patients. But in my case, it was clear that one or more of the controls in the so called “swiss cheese model” of protective measures failed to prevent a contamination.
As someone who grew up in the UK pharmaceutical industry and later joined MHRA as an inspector, I learned even in 1977 not long out of university, that steam quality was important. In the UK at that time, there were government issued technical memoranda for hospital Central Sterile Supplies Departments (CSSDs) requiring periodic steam quality testing. These were listed in a guidance called HTM 10 first published in 1980 and later updated to HTM 2010 in 1998 and again in 2012 when it was replaced by EN and ISO documents. That said the withdrawn HTM2010 none the less is a good source of intelligence on how to manage sterilisers in your pharmaceutical quality system. These initially UK requirements were later adopted with minor revision into European Norms and later ISO standards, and were used as reference standards by UK and European medicines inspectors, as well as those in countries such as Canada, Singapore, Australia, and New Zealand who had often translated these UK national standards into their national controls for hospital CSSDs.
To find some 40 years later that we still find some companies outside of Europe and the USA still ignorant of, or at least not adhering to these norms is a serious indictment of the insularity of many in the pharmaceutical industry, especially those hoping to export their products, and those who regulate it.
What are the current regulatory expectations?
The expectations are best described in the current WHO/PICs/EU revision of the sterile product GMP guidance. They are referenced in earlier versions of the GMP though less explicitly. Version 12 of the revision states:
"6.17 Steam used as a direct sterilizing agent should be of suitable quality and should not contain additives at a level which could cause contamination of product or equipment.
For a pure steam generator supplying pure steam used for the direct sterilization of materials or product-contact surfaces (e.g., porous hard-good autoclave loads), steam condensate should meet the current monograph for WFI of the relevant Pharmacopeia. A suitable sampling schedule should be in place to ensure that representative pure steam samples are obtained for analysis on a regular basis. Other aspects of the quality of pure steam used for sterilization should be assessed periodically against validated parameters. These parameters should include the following: non-condensable gases, dryness value (dryness fraction) and superheat."
EN285:2006+ A2:2009
The GMP draft does not give guidance values or test methods for these criteria but EN 285, the European Norm for "Large Steam Sterilizers" [1] does and is the standard that inspectors will expect companies to adopt.

EN 285:2015 is the world's baseline guidance for steam quality acceptance criteria. It is referenced in most national standards and in ISO 17665 [2].
Intended for large sterilisers (autoclaves) > 60L
• Compliance with the latest standard was required by Nov 2009 for all member states of the CEN (European Committee for Standardisation).
With the release of EN 285:2015, the bar has been raised. The acceptance criteria are shown in the following table.

Of course, companies could propose alternative tests and limits for these criteria but expect a long, difficult, and protracted discussion with the regulators and your company will have to demonstrate that their expertise and knowledge is at least equivalent to the Technical Committee of experts that prepared the norms!
Note: Alternative procedures can be used providing they has been calibrated against the European Standard.
Why do the current regulatory expectations exist and what do companies need to be doing?
The quality of the steam supplied via the distribution system, to an equipment for sterilisation is an important factor in steam sterilization. Time, temperature, and pressure, and evacuation or other load air removal steps, are critical variables in the success and consistency of any sterilization process. These general requirements are not exclusive to steam sterilisers (autoclaves) but apply also to automated stopper washer-sterilisers as well as in-situ SIP systems for aseptic processing equipment.
The principles should also be applied to the sterilisation of fermenters and bioreactors where reliable decontamination is mandated, but in these last cases the patient impact may be less, and any contamination could be primarily a production loss to the company (which could be considerable!).
The importance of steam quality must therefore be included in the relevant process and equipment risk assessments e.g., autoclave and process FMEA, and depending upon cycle design and purpose of the sterilising cycle an assessment of steam quality should be one part of the contamination control strategy (CCS) and validation of any steam sterilizer should generate evidence of control both in initial validations but subsequently on an ongoing basis.
It must, however, be emphasized that steam quality measurements are just one (and the last) point of control and as always in GMP real world consistency comes from good design of the generation and distribution system and its routine maintenance and operation and never usage point testing alone.
In GMP, Steam quality is defined as the measurable physical and chemical attributes of steam used for sterilization. Some authors use the term only for physical parameters, but this is more a matter of regional practice rather than global consistency. (There are steam quality expectations in other industries too! Expectations are in fact quite similar, as thermal efficiency and premature failures are major risks e.g., where steam turbines are used in energy generation or in ships engines and as in many aspects of quality origins often have a basis in military equipment design and construction).
Chemical attributes
Where the steam directly contacts product contact surfaces such as equipment, whether wrapped or not, or product components such as rubber stoppers, the steam must not leave chemical residues. It is for this reason it is expected that steam condensate should meet the requirements for Water for Injection (WFI).
Physical attributes
These physical attributes and variables include temperature (superheat), dryness (liquid water content), and non-condensable gas content (air, nitrogen, and occasionally CO2.
Deviations from established ranges of these physical aspects of the steam can result in the following issues:
• Wet loads
• Damaged loads
• Unsterile loads
• Sterilization (biological and chemical) indicator failures
• Staining and corrosion of instruments and containers
• Premature failure of valves, steam traps and erosion of pipework and valve seals in the distribution system
Each of these issues has a specific cause or a combination of causes and can usually be remedied by good design and maintenance.
What QA needs to "Know About Steam Quality"
Most sterilizer manufacturers recommend “97% pure steam” in their technical literature as a services specification. In general, this is often not well defined, and rarely measured.
Fortunately, unlike the days when the UK hospital HTM 10 standards were published, essentially all laboratory and production autoclaves on the market today can provide sterile, dry, and intact sterilization loads if provided good quality steam from the steam supply. The bad news is that any steam autoclave can experience the above problems, and the cause is not always something that can be easily predicted.
With careful design, following well-established principles, and proper maintenance, the system (steam supply and sterilizer) can be engineered to provide a large margin of security against steam quality noncompliance. For a production or GMP environment, steam quality testing should be part of annual preventative maintenance and qualification testing and should always be considered whenever there is a modification or major maintenance to an autoclave, steam distribution network, or steam generation system.
Steam Quality (SQ) Testing Methods and Acceptance Criteria, When steam quality testing is performed, three physical parameters are measured:
Steam Dryness: The amount of the steam by weight that is steam and not liquid water;
Non-condensable gases: The amount of the steam by volume that is not steam or water, but is air or other gas that does not contribute meaningfully to sterility of the load;
Superheat: The temperature of the steam above the temperature of saturated steam for a given moisture content;
Steam Dryness Testing
Steam dryness is calculated by measuring the temperature change in a known amount of water in relation to the mass of steam that is required to cause that temperature change. Ideally, the temperature rise is exactly proportional to the amount of steam delivered to the water to heat it, resulting in a dryness value of 1.0 (i.e., perfectly dry steam with no liquid water content.)
Normally, the dryness value is less than 1.0, as there are thermal losses in any piping system even if it is very well insulated. Because the dryness value of the steam at the chamber entry point can be quite a bit lower than the dryness value in the sterilizer, measurements of steam dryness should be made at both locations.
The acceptance criterion in EN 285 for steam dryness (the fraction of steam relative to water – 1.0 = all steam, no water) is at least 0.95, or 95% by weight for production sterilisers. A dryness level down to 90% is considered often considered acceptable for laboratory autoclaves e.g., in QC microbiology, however, steam below this value is wet steam.
Wet steam does not deliver as much energy to the load as >90% saturated steam and excessive condense on the surface of materials will act as an insulator due to the poor conductivity of water compared to other materials. It is therefore important to ensure that loads drain well and there is limited pooling of condensed water in the packs or items being sterilised.
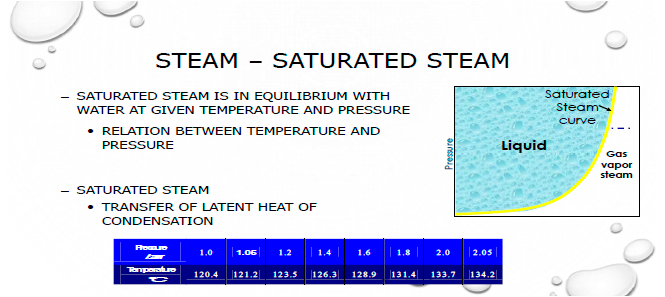
If the steam is wet, it can result in what is known as "wet packs". If the steam is wet, or if the saturation level has decreased since the last validation, the expected Sterility Assurance Level will probably not be achieved. This is especially important for bioburden-based validations since overkill cycles have more of a safety margin by their design, but failures can and have occurred even in overkill cycles where wet steam combines with poor air removal to generate steam penetration and poor heat transfer.
Wet loads can also result from other factors such as wrapping items too tightly or having a poorly designed load that causes normal condense to pool in the load and not drain freely.
Common production related to damp clean room gowns and problems due to damp stoppers jamming in rubber stopper guides and clumping in sterilisation bags with sometimes BI survivors where sterility assurance calculations suggest there should be no problem. Often companies try to remedy the situation by extended drying cycles rather than addressing the true root cause or combined causes that lead to the error train. See the section on non-compliant results below.
If the wet steam is due to ineffective droplet ejection in the steam generator, then it is possible for the droplets of water from the generator hot well to be contaminated with endotoxin concentrated from the feed water.
Non-condensable gases (NCG)
Non-condensable gases are generally air and air is a poor sterilant compared to steam.
As an example, a typical dry-heat sterilization exposure phase lasts upwards of two hours at a temperature of at least 160°C/320°F. Steam sterilization typically is done with exposure phases of 15 minutes at 121°C/250°F or 3.5 minutes at 134°C.
Non-condensable gases decrease sterilization efficacy. As with wet steam, the Sterility Assurance Level will be less than expected if non-condensable gas content has increased since product sterility validation. The percentage of non-condensable gases in the steam should be less than or equal to 3.5% by volume.
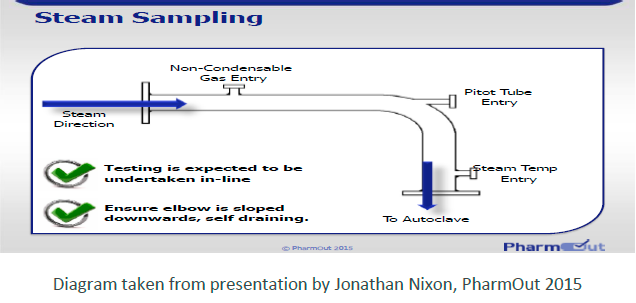
Air within a sterilisation load comes from three sources:
• Air carried into the chamber with NCGs in the steam
• Air not removed from the chamber during the initial part of the cycle for example by inefficient vacuum pulsing at the start of a "porous or hard surface load cycle" or ineffective bleeding of steam by downward displacement in a “fluids load” or air leaking into the chamber during sub atmospheric vacuum pulsing.
• Air not removed from the load itself due to entrapment e.g., due to incorrect sizing of vent filters
The non-condensable bas tests are designed to detect the first bulleted problem above only.
Superheat
The steam is sampled in free expansion into ambient air. The maximum temperature measured at a precise location in the jet is the temperature upon which the superheat analysis is based. When the temperature and moisture content do not match up, two things can happen:
1) If the moisture content is higher than saturation for the temperature, wet loads occur, as discussed previously.
2) When the moisture content is lower than saturation for the temperature, the condition is called superheat.
In superheat, the steam is too dry, and its energy content is too high. When the steam condenses on the load, the energy released is enough to melt plastic packaging and char paper packaging. Neither is a good outcome.
If your Tyvek overwrapped items show signs of distortion or melting, superheated steam may be one of the potential root causes.
What causes non-compliant results?
Wet steam
• Inadequate insulation around the sterilizer or steam piping, allowing energy loss and condensation in the distribution system
• Poorly controlled steam boiler chemistry (especially a deficiency of sulphites)
• Low sections of piping between the boiler and the sterilizer, allowing condensate to pool and be carried over with the steam entering the chamber. A suitable number of steam traps should be installed not just at the autoclave but also in the steam distribution network
• Too great a pressure drop across a regulator or between the jacket and chamber, which causes the “extra” water in the steam at the higher pressure to fall out as condensate
• No/clogged steam filters, either letting condensate pass if no filter, or causing a pressure drop that causes condensate to fall out
• No/clogged steam traps/separators, in either case, condensate in the steam line is not removed
• Steam trap/filter too far from the sterilizer, allowing condensate to be generated between the trap or filter and the sterilizer

• Inadequate number of steam traps for the distance that steam must travel from its source to the sterilizer
• Bad steam system design (vertical drops of steam direct to the sterilizer, no traps, no header, etc.)
• Load too dense/too cold when placed in sterilizer
• Foaming of the water in the boiler
• Inadequate blow down of the steam generator hot well
• Explosive boiling in the steam generation system due to faulty level control devices in the hot well of the steam generator.
• Poorly designed droplet ejection devices on the pure steam generator
Non-condensable gases (NCGs)
NCGs are brought into the steam primarily via two sources:
• Leaks/cracks in the steam plumbing, filters, separators, etc.
• Inadequate deaeration of the boiler feed water
NB. Air not removed from the chamber during the initial part of the cycle for example by inefficient vacuum pulsing at the start of a “porous or hard surface load cycle” or ineffective bleeding of steam by downward displacement in a “fluids load” or air leaking into the chamber during sub atmospheric vacuum pulsing. Additionally, air not removed from the load itself due to entrapment e.g., due to incorrect sizing of vent filters are other air in chamber problems not linked to the NCG measurement of the chamber steam supply but in poorly designed cycles these problems may also be exacerbated by additional air contaminating the steam entering the chamber
Superheat
Superheat can result from the following sources:
• Jacket temperature/pressure too high
• Steam pressure/temperature too high entering the sterilizer
• Steam flowing through a small orifice or tight-radiused direction change between its source and the chamber causing a large pressure reduction/steam velocity increase
The temperature shown on the sterilizer controls generally will not show superheat values, even if superheat is present. Since the temperature is measured in the drain of the sterilizer chamber, superheat will have been dissipated into the load, chamber wall, door, and bulkhead long before it reaches that sensor.
Solutions
Each of the steam quality parameters can be measured and, if issues arise, addressed.
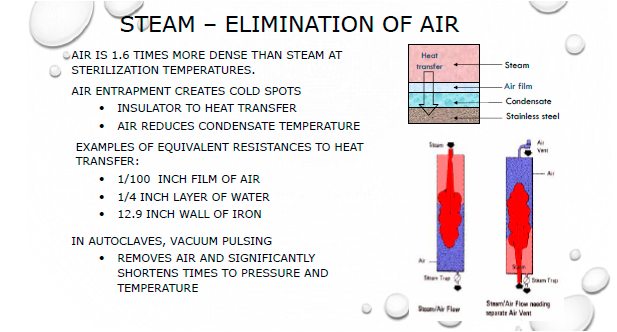
When should testing be performed?
The first step is to measure, even if there are no problems. This should be done on a regular basis — at initial installation, and after preventative maintenance to establish a baseline for the system. Measurements made when there are no problems can also provide an indication if the sterilizer is close to having a problem. Measurements should also be made when changes are made to supply plumbing.
The frequency of testing should be according to robust risk assessment by a knowledgeable team and the justification documented.
EN285 does not specify requirements for validation and routine control of sterilisation by moisture heat. (1)
However, WHO/EU/PICs GMP states “All sterilisation processes should be validated” and “The validity of the process should be verified at scheduled intervals, at least annually and whenever significant modifications have been made to the equipment.”
ISPE Good Practice Guide: Commissioning and Qualification of Pharmaceutical Water and Steam Systems. Appendix 2 - Section 2.3.1 suggests for initial validation of a Pure Steam Generator that the System is split into Phase 1 & Phase 2:
Phase 1: (Start-up) approximately three days – sample each point of use at least once. Sample each pure steam generator outlet at least once.
Phase 2: (system consistency / stability) approximately one week – sample each point of use at least once. Sample each generator more than once.
Risk assessment of the control of clean steam supplies
The risk assessment prior to validation should consider, as a minimum:
1. What previous experience does the company have with its autoclaves / steam distribution systems?
2. How long does the distribution system take to warm up following start-up?
3. When is the autoclave able to start?
4. Are there any other users of the steam system?
5. Are multiple users able to operate at the same time?
6. What is the maximum demand that can be placed on the steam system?
7. Are there other demands on the feed water that could affect the quality of the feed water?
Where can I get information and test kits to facilitate steam quality testing?
Much of the information in this paper has been gathered over years of industry practice and the attendance of a formal steriliser courses including a trouble shooting course for steriliser users when I was an inspector with MHRA.
These courses were organised and conducted by an expert of very many years, a gentleman called Mr Keith Shuttleworth.
If you are involved with any sort of Clean Steam Testing, then I would certainly recommend his test kit and utilising the knowledge provided on his website.

• www.ksapharma.com
• info@ksapharma.com
References
[1] EN 285, “Sterilization. Steam sterilizers. Large sterilizers”, CEN, national Standards Making Bodies.
[2] ISO 17665-1, “, Sterilization of health care products – Moist heat – Part1: Requirements for the development, validation, and routine control of a sterilization process for medical devices,” ISO, Geneva, Switzerland, 2006.
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:
